…Boosters required for vaccine protection after 1 year
By Sola Ogundipe
The World Health Organization (WHO) has approved the first mpox diagnostic test for emergency use, significantly boosting the diagnostic capacity in countries grappling with outbreaks of the disease.
The test, known as the Alinity m MPXV assay, is a real-time polymerase chain reaction (PCR) test that detects monkeypox virus (MPXV) DNA from human skin lesion swabs. Manufactured by Abbott Molecular Inc., an American company, the test is designed for use by trained clinical laboratory personnel.
“This first mpox diagnostic test listed under the Emergency Use Listing procedure represents a significant milestone in expanding testing availability in affected countries,” said Dr. Yukiko Nakatani, WHO Assistant Director-General for Access to Medicines and Health Products.
PCR testing, which detects viral DNA, remains the gold standard for diagnosing mpox. Early diagnosis is critical for enabling timely treatment and controlling the virus, though limited testing capacity and delays in confirming cases continue to hinder efforts in Africa, where mpox remains widespread.
Over 30,000 suspected mpox cases have been reported in Africa this year, with the highest numbers in the Democratic Republic of the Congo (DRC), Burundi, and Nigeria. Notably, only 37% of suspected cases in the DRC have been tested.
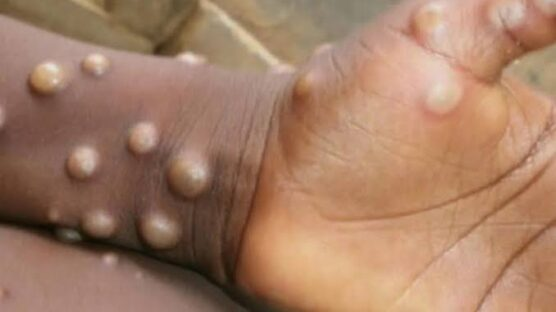
Mpox, formerly known as monkeypox, is an infectious disease caused by the MPXV. Symptoms include a painful rash, swollen lymph nodes, fever, headaches, and muscle aches.
Africa has experienced an unprecedented rise in mpox cases this year, driven by two distinct strains of the virus. Transmission is predominantly occurring in the DRC.
WHO’s Emergency Use Listing (EUL) procedure accelerates the availability of life-saving medical products, such as vaccines, diagnostics, and treatments, in the context of a Public Health Emergency of International Concern (PHEIC). In August, WHO urged manufacturers to submit expressions of interest for EUL to address the urgent need for increased testing capacity as the virus continued to spread.
So far, WHO has received three additional submissions for EUL evaluation, and discussions are ongoing with other manufacturers.
The DRC, at the center of the mpox epidemic, has launched a vaccination campaign in the eastern city of Goma. The launch, originally scheduled for last Wednesday, was delayed due to logistical challenges. The first doses were administered to healthcare workers, with the general population set to receive vaccinations starting Monday in the eastern region, where the current outbreak began a year ago. The DRC has so far received 265,000 vaccine doses and is awaiting millions more promised by France, Japan, and the United States.
Mpox Vaccine Requires Boosters After One Year, Study Finds
A new study has found that the mpox vaccine requires booster doses after approximately one year for continued protection.
Dr. Ai-ris Yonekura Collier, co-director of the Clinical Trials Unit at Beth Israel Deaconess Medical Center (BIDMC) in Boston, stressed the importance of ensuring broad access to the full vaccine series. The study, published in the Journal of the American Medical Association, suggests that individuals vaccinated during the 2022 outbreak need booster shots to maintain immunity.
The research tracked immune responses over 12 months in 45 individuals who were either vaccinated during the outbreak or had a confirmed diagnosis of mpox. Participants had received one or two doses of the Jynneos mpox vaccine. Researchers measured antibody and T-cell responses initially, at three weeks, and every three months for a year. The study found that vaccine-generated protection waned significantly within six to 12 months.
Public health officials prioritized vaccination during the 2022 outbreak for high-risk groups, including people with new or multiple sex partners, men who have sex with men, healthcare workers, lab personnel, and travelers to areas where the virus was present.
Dr. Dan Barouch, director of BIDMC’s Center for Vaccine and Virology Research, emphasized the need for larger human studies to assess the long-term effectiveness of the vaccine.
Disclaimer
Comments expressed here do not reflect the opinions of Vanguard newspapers or any employee thereof.